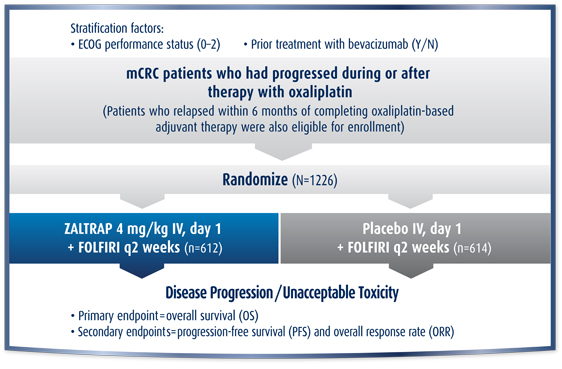
VELOUR Study Design1
The efficacy and safety of ZALTRAP were evaluated in a randomized (1:1), double-blind, placebo-controlled study in patients with metastatic colorectal cancer (mCRC) who were resistant to or had progressed following an oxaliplatin-containing regimen, with or without prior bevacizumab.1
ECOG=Eastern Cooperative Oncology Group.
FOLFIRI: irinotecan 180 mg/m2 intravenously over 90 minutes and leucovorin (dl racemic) 400 mg/m2 intravenously over 2 hours at the same time on day 1 using a Y-line, followed by fluorouracil 400 mg/m2 as an intravenous bolus, and then by fluorouracil 2400 mg/m2 as a continuous intravenous infusion over 46 hours1
- Entry criteria included:
- Patients who progressed during or following an oxaliplatin-based chemotherapy for metastatic disease were eligible
- Patients who relapsed within 6 months of completing oxaliplatin-based adjuvant chemotherapy were eligible
ZALTRAP (ziv-aflibercept)
INDICATION
ZALTRAP®, in combination with fluorouracil, leucovorin, irinotecan-(FOLFIRI), is indicated for the treatment of patients with metastatic colorectal cancer (mCRC) that is resistant to or has progressed following an oxaliplatin-containing regimen.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
-
Hemorrhage: Patients treated with ZALTRAP have an increased risk of hemorrhage, including severe and sometimes fatal hemorrhagic events.
- Bleeding/hemorrhage (all grades) was reported in 38% of ZALTRAP/FOLFIRI patients vs. 19% of placebo/FOLFIRI patients. Grade 3-4 hemorrhagic events, including GI hemorrhage, hematuria, and post-procedural hemorrhage, occurred in 3% of ZALTRAP/FOLFIRI patients vs. 1% of placebo/FOLFIRI patients. Severe intracranial hemorrhage and pulmonary hemorrhage/hemoptysis including fatal events have occurred in patients receiving ZALTRAP.
- Monitor patients for signs and symptoms of bleeding. Do not initiate ZALTRAP in patients with severe hemorrhage. Discontinue ZALTRAP in patients who develop severe hemorrhage.
-
Gastrointestinal Perforation: GI perforation including fatal GI perforation can occur in patients receiving ZALTRAP.
- Across three clinical trials (colorectal, pancreatic, and lung cancer), GI perforation (all grades/Grade 3-4) occurred in 0.8% /0.8% of Zaltrap patients and 0.3% /0.2% for placebo patients.
- Monitor patients for signs and symptoms of GI perforation. Discontinue ZALTRAP in patients who experience GI perforation.
-
Impaired Wound Healing: Grade 3 impaired wound healing was reported in 2 patients (0.3%) treated with ZALTRAP/FOLFIRI.
- Discontinue ZALTRAP in patients with impaired wound healing. The safety of resumption of ZALTRAP after resolution of wound healing complications has not been established.
- Withhold ZALTRAP for at least 4 weeks prior to elective surgery and do not administer ZALTRAP for at least 4 weeks after major surgery and until wounds have adequately healed.
- For minor surgery such as central venous access port placement, biopsy, and tooth extraction, ZALTRAP may be initiated/resumed once the surgical wound is fully healed.
- Fistula Formation: Fistula formation involving GI and non-GI sites occurs at a higher incidence in patients treated with ZALTRAP. Fistulas (anal, enterovesical, enterocutaneous, colovaginal, intestinal sites) were reported in 1.5% (9/611) of ZALTRAP/FOLFIRI treated patients and 0.5% (3/605) of placebo/FOLFIRI patients. Grade 3 GI fistula formation occurred in 2 patients treated with Zaltrap (0.3%) and 1 patient treated with placebo (0.2%). Discontinue ZALTRAP therapy in patients who develop fistula.
-
Hypertension: An increased risk of Grade 3-4 hypertension has been observed in patients receiving ZALTRAP.
- There is no clinical trial experience administering ZALTRAP to patients with NYHA class III or IV heart failure. In patients with mCRC, Grade 3 hypertension (defined as requiring adjustment in existing antihypertensive therapy or treatment with more than one drug) was reported in 1.5% of patients treated with placebo/FOLFIRI and 19% treated with ZALTRAP/FOLFIRI. Grade 4 hypertension (hypertensive crisis) was reported in 1 patient (0.2%) treated with ZALTRAP/FOLFIRI. Of patients treated with ZALTRAP/FOLFIRI who developed Grade 3-4 hypertension, 54% had onset during the first two cycles of treatment.
- Monitor blood pressure at least every two weeks, treat with appropriate antihypertensive therapy, and continue monitoring blood pressure regularly during ZALTRAP treatment. Temporarily suspend ZALTRAP until hypertension is controlled, and permanently reduce the ZALTRAP dose to 2 mg/kg for subsequent cycles. Discontinue ZALTRAP in patients with hypertensive crisis or hypertensive encephalopathy.
- Arterial Thromboembolic Events: Arterial thromboembolic events (ATE), including transient ischemic attack, cerebrovascular accident, and angina pectoris, occurred more frequently in patients who have received ZALTRAP. ATE occurred in 2.6% of ZALTRAP/FOLFIRI patients and 1.7% of placebo/FOLFIRI patients. Grade 3-4 events occurred in 11 patients (1.8%) treated with ZALTRAP/FOLFIRI and 4 patients (0.7%) treated with placebo/FOLFIRI. Discontinue ZALTRAP in patients who experience an ATE.
-
Proteinuria: Severe proteinuria, nephrotic syndrome, and thrombotic microangiopathy (TMA) occurred more frequently in patients treated with ZALTRAP.
- Proteinuria was reported in 62% of ZALTRAP/FOLFIRI patients compared to 41% of placebo/FOLFIRI patients. Grade 3-4 proteinuria occurred in 8% of ZALTRAP/FOLFIRI patients compared to 1% of placebo/FOLFIRI patients. Nephrotic syndrome occurred in 2 patients (0.5%) treated with ZALTRAP/FOLFIRI compared to none of the patients treated with placebo/FOLFIRI. TMA was reported in 3 of 2,258 patients with cancer enrolled across completed studies.
- Monitor proteinuria by urine dipstick analysis and/or urinary protein creatinine ratio (UPCR) for the development or worsening of proteinuria. Obtain a 24-hour urine collection in patients with a dipstick of ≥2+ for protein or UPCR >1
- Suspend ZALTRAP when proteinuria ≥2 grams/24 hours and resume ZALTRAP when proteinuria <2 grams/24 hours.
- If recurrent, suspend until proteinuria <2 grams/24 hours and then reduce ZALTRAP dose to 2 mg/kg.
- Discontinue ZALTRAP if nephrotic syndrome or TMA develops.
-
Neutropenia and Neutropenic Complications: A higher incidence of neutropenic complications (febrile neutropenia and neutropenic infection) occurred in patients receiving ZALTRAP.
- Grade 3-4 neutropenia occurred in 37% of ZALTRAP/FOLFIRI patients compared to 30% of placebo/FOLFIRI patients. Grade 3-4 febrile neutropenia occurred in 4% of ZALTRAP/FOLFIRI patients compared to 2% of placebo/FOLFIRI patients. Grade 3-4 neutropenic infection/sepsis occurred in 1.5% of ZALTRAP/FOLFIRI patients compared to 1.2% of placebo/FOLFIRI patients.
- Monitor CBC with differential count at baseline and prior to initiation of each cycle of ZALTRAP. Delay administration of ZALTRAP/FOLFIRI until neutrophil count is ≥ 1.5 x 109/L.
-
Diarrhea and Dehydration: Incidence of severe diarrhea and dehydration is increased in patients treated with ZALTRAP/FOLFIRI.
- Grade 3-4 diarrhea was reported in 19% of ZALTRAP/FOLFIRI patients compared to 8% of placebo/FOLFIRI patients. Grade 3-4 dehydration was reported in 4% of ZALTRAP/FOLFIRI patients compared to 1% of placebo/FOLFIRI patients.
- The incidence of diarrhea is increased in patients ≥65 years of age compared to patients <65 years of age. Monitor closely.
- Reversible Posterior Leukoencephalopathy Syndrome: RPLS (also known as posterior reversible encephalopathy syndrome) was reported in 0.5% of 3795 patients treated with ZALTRAP monotherapy or in combination with chemotherapy. Confirm diagnosis of RPLS with magnetic resonance imaging (MRI) and discontinue ZALTRAP in patients who develop RPLS. Symptoms usually resolve or improve within days, although some patients have experienced ongoing neurologic sequelae or death.
- Embryo-Fetal Toxicity: Based on findings from animal studies and its mechanism of action, ZALTRAP can cause fetal harm when administered to pregnant women. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with ZALTRAP and for 3 months following the last dose.
ADVERSE REACTIONS
- The most common adverse reactions (≥20%) in the ZALTRAP/FOLFIRI arm were leukopenia, diarrhea, neutropenia, proteinuria, AST increased, stomatitis, fatigue, thrombocytopenia, ALT increased, hypertension, weight decreased, decreased appetite, epistaxis, abdominal pain, dysphonia, serum creatinine increased, and headache.
- The most common Grade 3-4 adverse reactions (≥5%) in the ZALTRAP/FOLFIRI arm were neutropenia, diarrhea, hypertension, leukopenia, stomatitis, fatigue, proteinuria, and asthenia.
- Infections occurred at a higher frequency in patients receiving ZALTRAP/FOLFIRI (46%, all grades; 12%, Grade 3-4) than in patients receiving placebo/FOLFIRI (33%, all grades; 7%, Grade 3-4), including urinary tract infection, nasopharyngitis, upper respiratory tract infection, pneumonia, catheter site infection, and tooth infection.
- In patients with mCRC, venous thromboembolic events (VTE), consisting primarily of deep venous thrombosis and pulmonary embolism, occurred in 9% of patients treated with ZALTRAP/FOLFIRI and 7% of patients treated with placebo/FOLFIRI.
USE IN SPECIFIC POPULATIONS
- Pregnancy: Based on findings from animal reproduction studies and its mechanism of action, ZALTRAP can cause fetal harm when administered to pregnant women. There is insufficient data in pregnant women exposed to ZALTRAP to assess the risks. Advise pregnant women of the potential risk to a fetus.
- Lactation: There are no data on the presence of ZALTRAP in human milk, or the effects of ZALTRAP on the breastfed infant or on milk production. Because of the potential for serious adverse reactions in breastfed infants, advise women not to breastfeed during treatment with ZALTRAP and for 1 month following the last dose.
- Females and Males of Reproductive Potential: Verify the pregnancy status in females of reproductive potential prior to initiating ZALTRAP. Advise female patients of reproductive potential to use effective contraception during treatment with ZALTRAP and for 3 months following the last dose. Advise female and male patients of reproductive potential that ZALTRAP may impair reproductive function and fertility.
Please see full Prescribing Information.
- References:
- 1. ZALTRAP Prescribing Information. Bridgewater, NJ: sanofi-aventis U.S. LLC.
- 2. Tew WP, Gordon M, Murren J, et al. Clin Cancer Res. 2010;16:358-366.
- 3. Riely GJ, Miller VA. Clin Cancer Res. 2007;13(15 suppl):4623s-4627s.